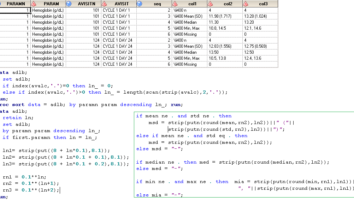
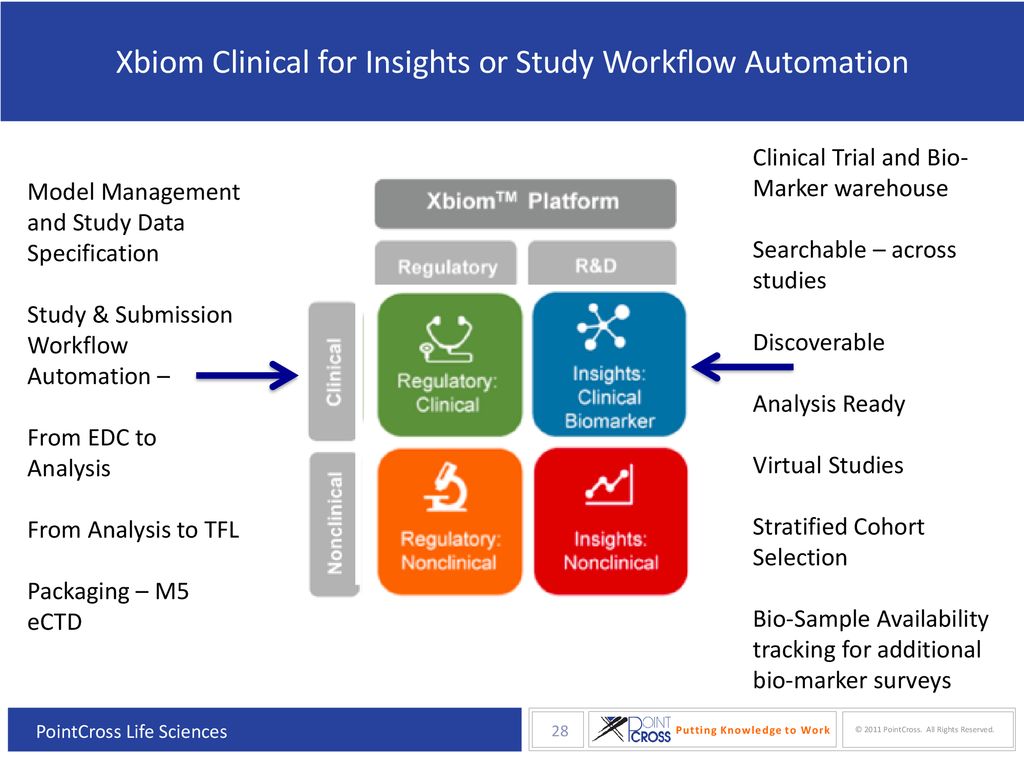
Large-scale TFL Automation for regulated Pharmaceutical trials using CDISC Analysis Results Metatadata
CLINICAL STUDY REPORT - IN-TEXT TABLES, TABLES FIGURES AND GRAPHS, PATIENT AND INDIVIDUAL PATIENT DATA LISTINGS: ICH E3 TECHNIC
![PDF] Come Out of Your Shell: A Dynamic Approach to Shell Implementation in Table and Listing Programs | Semantic Scholar PDF] Come Out of Your Shell: A Dynamic Approach to Shell Implementation in Table and Listing Programs | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/002f3667ffac04a1fa7ca0248b2270413ad354be/3-Table1-1.png)
PDF] Come Out of Your Shell: A Dynamic Approach to Shell Implementation in Table and Listing Programs | Semantic Scholar

R/Pharma 2021 Day 3. Rikke Nørmark Mortensen. Auto generation of PowerPoint for clinical trials - YouTube
![PDF] Come Out of Your Shell: A Dynamic Approach to Shell Implementation in Table and Listing Programs | Semantic Scholar PDF] Come Out of Your Shell: A Dynamic Approach to Shell Implementation in Table and Listing Programs | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/002f3667ffac04a1fa7ca0248b2270413ad354be/12-Table2-1.png)
PDF] Come Out of Your Shell: A Dynamic Approach to Shell Implementation in Table and Listing Programs | Semantic Scholar
Large-scale TFL Automation for regulated Pharmaceutical trials using CDISC Analysis Results Metatadata
CLINICAL STUDY REPORT - IN-TEXT TABLES, TABLES FIGURES AND GRAPHS, PATIENT AND INDIVIDUAL PATIENT DATA LISTINGS: ICH E3 TECHNIC
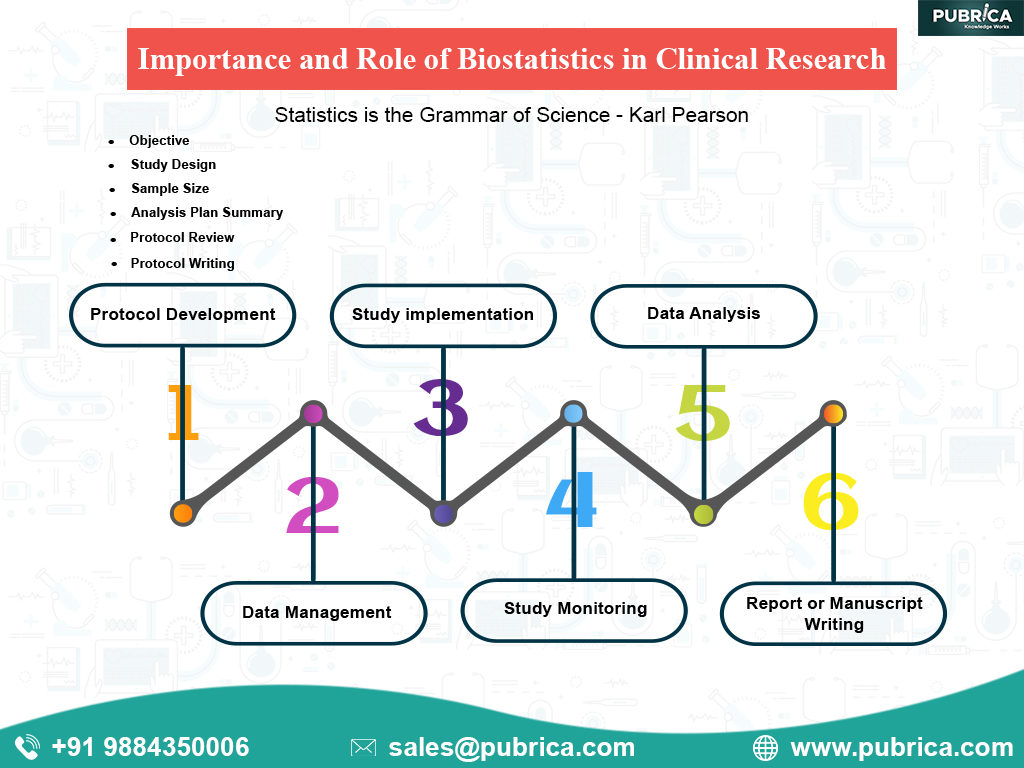
Biostatistical Programming Services For Clinical trials study data management, cro Statistical Analysis, Medical Report audit help in Uk, Usa and Australia
CLINICAL STUDY REPORT - IN-TEXT TABLES, TABLES FIGURES AND GRAPHS, PATIENT AND INDIVIDUAL PATIENT DATA LISTINGS: ICH E3 TECHNIC
Large-scale TFL Automation for regulated Pharmaceutical trials using CDISC Analysis Results Metatadata